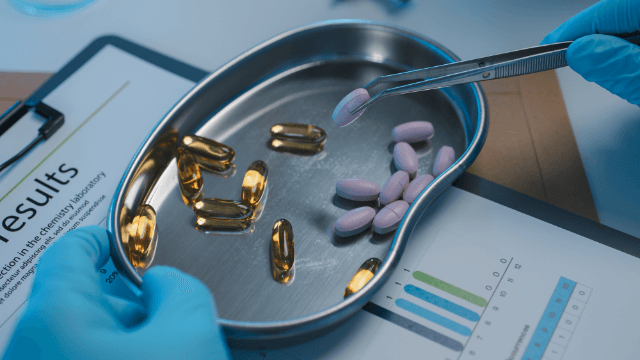
Quality Framework
Our facility and processes are designed from the ground up to meet one of the strictest global regulatory frameworks, GMP. We are committed to product safety, strict hygiene protocols and ingredient integrity – protecting you and your customers from poor quality and unsafe products.

Quality Framework
The Quality Framework at PharmaNZ has been developed based on the PIC/S guidelines (Pharmaceuticals Inspection Co-operation Scheme) and Code of GMP (Good Manufacturing Practice) in conjunction with the legislation requirements of the Dietary Supplements Regulations 1985 and is regularly reviewed to enhance and embrace a continuous improvement and compliance culture.
Quality at the highest level
PharmaNZ follows strict GMP cleaning and hygiene protocols, including:
- Change barrier and redline practices
- Employee medical assessments
- Strict controls on material movement between warehouse and production through airlocks.
Certifications
We work hard to ensure we have the licenses and certificates required to get your product to market.







Quality is ingrained within PharmaNZ.
We build and implement robust, controlled and systematic practices to maintain the integrity of our products.
Our Facility
Our GMP facility currently covers a 2,500m2 footprint with a cleanroom area of 350m2.
The entire production facility is pressure controlled and contains separate change facilities, seven production rooms, one blending room, a staging area, a separate packing hall and an airlock to our large warehousing area.
PharmaNZ follows Good Manufacturing Practice (GMP) processes throughout our facility.